~ 4 ~
~ The Study of Threes ~
http://threesology.org
Researchers as of 8/29/2019
| Devil's Advocate Series: | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| 10 | 11 | 12 | 13 | 14A 14B |
15 | 16 | 17 | 18 |
| 19 | 20 | 21 | 22A 22B |
23 | 24 | 25 | 26 | 27 |
| 28 | 29 | 30 A | 30 B | 31 | 32 | 33a | 33b | 33c |
| 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 A | 41 B |
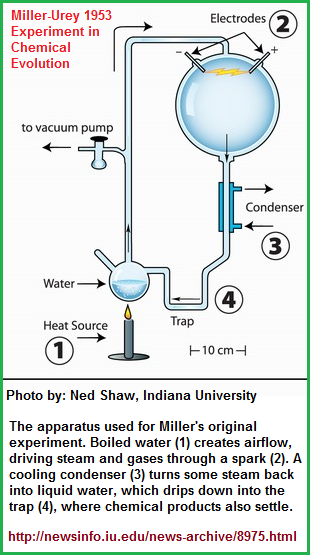
If you can visualize putting together a puzzle... for example a biological puzzle (after you have identified the pieces); one variable may well be to account for the height, width and depth at which oxygen reaches its outer edges... that is an analogy to the recurring singular mental tactic used quite often by many people by putting a conventional landscape or (static) multi-item portrait... (that is until we take into consideration the effects of solar irradiation and lightning as part of life's assumed combustion processes). Aside from the basic chemical elements, there are three forms of energy (solar irradiation, lightning, thermal venting) that appear to be given the top priority when experimentation into chemical evolution is proposed and conducted, such as for example the old Miller/Urey experiment in which electrical discharges were used to simulate an early pre-and beginning biotic environment as a means that of stimulating growth, much in the manner that electrical discharge from lighting was used in some old movies of Frankenstein. Who knows, perhaps the experiments called God was a mad scientist who created a life form that many think is akin to a monster, or monstrosity because of all the pain, anguish and suffering that has been produced by human activity.. Anyway, we now need to explore how deep both water and oxygen can go into the Earth, as a means of attempting to ascertain whether life's beginnings has a zone in which it can and can not originate. Whereby our visualized puzzle pieces assume a boundary, just like enumerations do.
|
Just like on the land, there are also photo-synthetically active plants and bacteria in the ocean, the primary producers. Annually, they generate about the same amount of oxygen and fix as much carbon as all the land plants together. This is quite amazing. After all, the total living biomass in the ocean is only about one two-hundredth of that in the land plants. This means that primary pro-ducers in the ocean are around two hundred times more productive than land plants with respect to their mass. This reflects the high productivity of single-celled algae, which contain very little inactive biomass such as, for example, the heartwood in tree trunks. Photosynthetic production of oxygen is limited, however, to the uppermost, sunlit layer of the ocean. This only extends to a depth of around 100 metres and, because of the stable density layering of the ocean, it is largely separated from the enormous underlying volume of the deeper ocean. Moreover, most of the oxygen generated by the primary producers escapes into the atmosphere within a short time, and thus does not contribute to oxygen enrichment in the deep water column. This is because the near-surface water, which extends down to around 100 metres, is typically saturated with oxygen by the supply from the atmosphere, and thus cannot store additional oxygen from biological production. In the inner ocean, on the other hand, there is no source of oxygen. Oxygen enters the ocean in the surface water through contact with the atmosphere. From there the oxygen is then brought to greater depths through the sinking and circulation of water masses. These, in turn, are dynamic processes that are strongly affected by climatic conditions. (Chapter 1). Three factors ultimately determine how high the concentration of dissolved oxygen is at any given point within the ocean:
The present-day distribution of oxygen in the internal deep ocean is thus determined by a complicated and not fully understood interplay of water circulation and bio-logical productivity, which leads to oxygen consumption in the ocean's interior. Extensive measurements have shown that the highest oxygen concentrations are found at high latitudes, where the ocean is cold, especially well-mixed and ventilated. The mid-latitudes, by contrast, especially on the western coasts of the continents, are characterized by marked oxygen-deficient zones. The oxygen supply here is very weak due to the sluggish water circulation, and this is further compounded by Âelevated oxygen consumption due to high biological productivity. This leads to a situation where the oxygen is almost completely depleted in the depth range between 100 and 1000 metres. This situation is also observed in the northern Indian Ocean in the area of the Arabian Sea and the Bay of Bengal. Different groups of marine organisms react to the oxygen deficiency in completely different ways, because of the wide range of tolerance levels of different marine animals to oxygen-poor conditions. For instance, crustaceans and fish generally require higher oxygen concentrations than mussels or snails. The largest oceanic oxygen minimum zones, however, because of their extremely low concentrations, should be viewed primarily as natural dead zones for the higher organisms, and by no means as caused by humans. 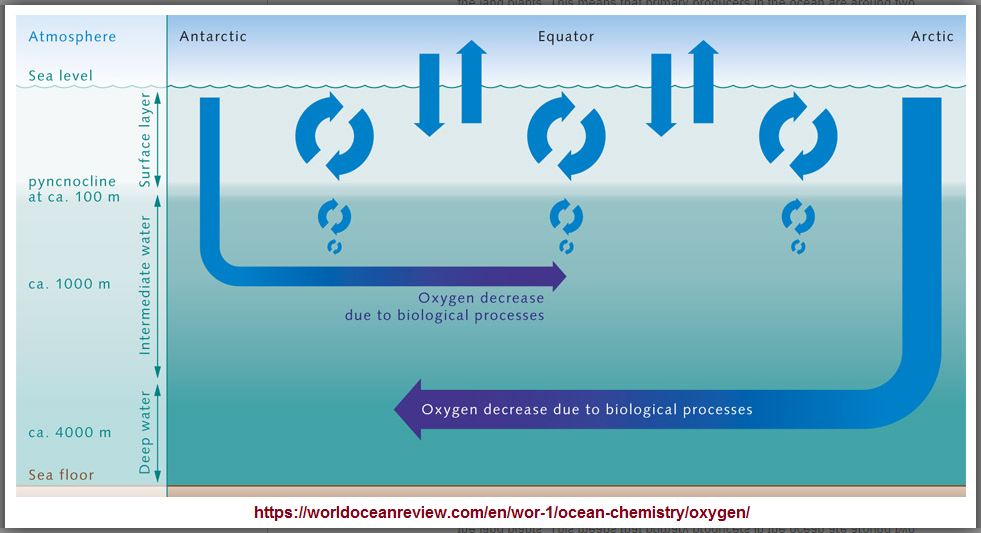 (Image 2.14) Oxygen from the atmosphere enters the near-surface waters of the ocean. This upper layer is well mixed, and is thus in chemical equilibrium with the atmosphere and rich in O2. It ends abruptly at the pyncnocline, which acts like a barrier. The oxygen rich water in the surface zone does not mix readily with deeper water layers. Oxygen essentially only enters the deeper ocean by the motion of water currents, especially with the formation of deep and intermediate waters in the polar regions. In the inner ocean, marine organisms consume oxygen. This creates a very sensitive equilibrium. 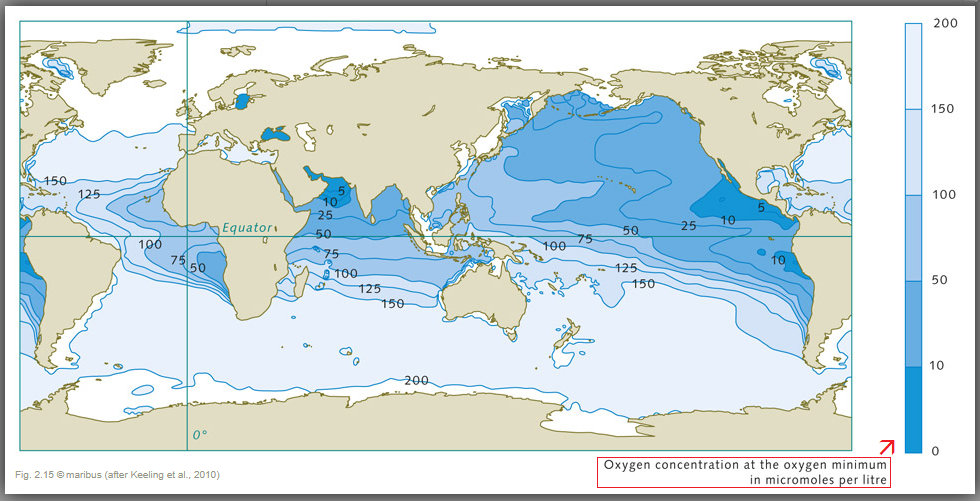 (Image 2.15) Marine regions with oxygen deficiencies are completely natural. These zones are mainly located in the mid-latitudes on the west sides of the continents. There is very little mixing here of the warm surface waters with the cold deep waters, so not much oxygen penetrates to greater depths. In addition, high bio-productivity and the resulting large amounts of sinking biomass here lead to strong oxygen consumption at depth, Âespecially between 100 and 1000 metres. |
If I may be permitted to digress for just a moment since the topic of continents came up and I want to include a reference to 3 major ideas about continents in the past that came to play a part in three associated ideas attributed to Darwin involving the topic of life with respect to Evolution; followed by three principals associated to Mendel's genetics:

We can summarize Darwin's theory of evolution through natural selection in three principles:
- Principle of variation. Among individuals within any population, there is variation in morphology, physiology, and behavior.
- Principle of heredity. Offspring resemble their parents more than they resemble unrelated individuals.
- Principle of selection. Some forms are more successful at surviving and reproducing than other forms in a given environment.
Mendel's Three Laws of Heredity are usually stated as:
- The Law of Segregation: Each inherited trait is defined by a gene pair. Parental genes are randomly separated to the sex cells so that sex cells contain only one gene of the pair. Offspring therefore inherit one genetic allele from each parent when sex cells unite in fertilization.
- The Law of Independent Assortment: Genes for different traits are sorted separately from one another so that the inheritance of one trait is not dependent on the inheritance of another.
- The Law of Dominance: An organism with alternate forms of a gene will express the form that is dominant.
And yet, as we continue on despite the momentary digression, we still do not have a clear picture of how deep... water goes nor if oxygen resides deeper into the Earth as well.... or if there exist processes which could have triggered their origination deep below the surface during the early formative years of the Earth . However, one of the problems encountered with the search for the origination of water and oxygen on Earth is when people ask leading questions such as "When did water come to Earth?" Such a question implies that water, if not oxygen and any number of elements and processes, did not evolve— nor were they residents already on the Earth as it formed. It's pretty simplistic thinking when we are inclined to think that a presumed absence billions of years ago in the form or formula that we now observe and describe, automatically means that a form or formula came from outside the Earth, if not the solar system and galaxy. Let's take a look at some differing perspectives, some of which are only concerned with the origination of water while others are concerned with the origination of oxygen and others take both into consideration... (whether or not they are cognizant of doing so, since oxygen exists in water connected with Hydrogen which is a molecule that can be split); as well as perhaps other forms and formulas into consideration:
- The Rise of Oxygen July 30th, 2003
Cyanobacteria or blue-green algae became the first microbes to produce oxygen by photosynthesis, perhaps as long ago as 3.5 billion years ago and certainly by 2.7 billion years ago. But, mysteriously, there was a long lag time – hundreds of millions of years – before Earth's atmosphere first gained significant amounts oxygen, some 2.4 billion to 2.3 billion years ago. Much hydrogen and oxygen both originated from water broken down by cyanobacteria. Cyanobacteria are microbes that live primarily in seawater. They are believed to have been the first organisms on Earth to perform oxygenic photosynthesis. In this process, they produce organic carbon, the building blocks of life's molecules, and release oxygen gas (O2). The O2 enters into the seawater, and from there some of it escapes into the atmosphere.
Oxygen has not always been as abundant as it is today. Most scientists believe that for half of Earth's 4.6-billion-year history, the atmosphere contained almost no oxygen. ...But Jim Kasting believes more of the oxygen produced by photosynthesis ended up buried within Earth's mantle, the layer beneath the crust, before the hydrogen could get to it. He is not sure how, but cites three theories:
- oxygen reacted with iron in seawater, and the resulting iron oxide precipitated onto the seafloor, then was buried deep within the Earth;
- oxygen-rich water in seafloor sediments was buried within the Earth, leaving oxygen in the mantle when the water's hydrogen was belched out by volcanoes;
- oxygen-rich sulfates in undersea hot springs reacted with iron in seafloor sediments, which were buried to put oxygen into the mantle.
- When Water Met Iron Deep Inside The Earth, It Might Have Created Conditions For Life by Carnegie Science, Monday, November 13, 2017
When the action of plate tectonics draws water-containing minerals down deep enough to meet the Earth's iron core, the extreme conditions cause the iron to grab oxygen atoms from the water molecules and set the hydrogen atoms free. The hydrogen escapes to the surface, but the oxygen gets trapped into crystalline iron dioxide, which can only exist under such intense pressures and temperatures.
- How did water come to Earth? by Brian Greene, May 2013
Here's the part we think we understand well: Just shy of a trillionth of a trillionth of a second after the Big Bang, the energy that sparked the outward swelling of space transmuted into a hot, uniform bath of particles. During the next three minutes, these primordial constituents bumped and jostled, combined and recombined, yielding the first atomic nuclei. One of the great triumphs of modern cosmology is its mathematical description of these processes, which gives accurate predictions for the cosmic abundances of the simplest nuclei" a lot of hydrogen, less helium and trace amounts of lithium. Producing copious hydrogen is a propitious start en route to water, but what about the other essential ingredient, oxygen?
That's where stars, already plentiful about a billion years after the Big Bang, enter the picture. Deep within their blisteringly hot interiors, stars are nuclear furnaces that fuse the Big Bang's simple nuclei into more complex elements, including carbon, nitrogen and, yes, oxygen. Later in their lives, when stars go super-nova, the explosions spew these elements into space. Oxygen and hydrogen commingle to make H2O.
So are we done? Not quite. In fact, this is where things get a little murky. Water molecules were surely part of the dusty swirl that coalesced into the Sun and its planets beginning about nine billion years after the Big Bang. But Earth's early history, including epochs with high ambient temperatures and no enveloping atmosphere, implies that surface water would have evaporated and drifted back into space. The water we encounter today, it seems, must have been delivered long after Earth formed.
- Planet Earth makes its own water from scratch deep in the mantle by Andy Goghlan 27 Jan 2017
...That's the upshot of a computer simulation of reactions in Earth's upper mantle between liquid hydrogen and quartz, the most common and stable form of silica in this part of the planet. "This is one way water can form on Earth," says team member John Tse at the University of Saskatchewan in Canada. "We show it's possible to have water forming in Earth's natural environment, rather than being of extraterrestrial origin." The simple reaction takes place at about 1400 °C and pressures 20,000 times higher than atmospheric pressure as silica, or silicon dioxide, reacts with liquid hydrogen to form liquid water and silicon hydride.
- NASA is planning to make water and oxygen on the Moon and Mars by 2020 by Sebastian Anthony on January 30, 2014 at 1:07 pm
NASA is forging ahead with plans to make water, oxygen, and hydrogen on the surface of the Moon and Mars. If we ever want to colonize other planets, it is vital that we find a way of extracting these vital gases and liquids from moons and planets, rather than transporting them from Earth (which is prohibitively expensive, due to Earth's gravity). The current plan is to land a rover on the Moon in 2018 that will try to extract hydrogen, water, and oxygen — and then hopefully, Curiosity's successor will try to convert the carbon dioxide in the atmosphere into oxygen in 2020 when it lands on Mars.
Granted that the foregoing selections do not actually give us distinct numerically- referenced depths of where oxygen and water exist in the Earth, they nonetheless provide indications that such resources may have originated at the inception of Earth's development billions of years ago. In other words, the actual numerical values with respect to the limits of depth is a matter of educated guess work since we are technologically unable to probe deep within the Earth. Nonetheless we have some semblance of a bottom (terrestrial) border for our puzzle.
Now that we have the assumed top and bottom, we must decide on the sides of the puzzle before addressing the many patterns within its borders which will enable us to begin creating some picture. For example, if we say that water and oxygen are products of other planetary places as well, this infers a Universe-wide zone of application which produces a very long panoramic view of a puzzle. Then again, if we simply limit our discussion to Earth, we nonetheless develop an image which creates three zones: An upper limit, a lower limit, and the zone limit in which life processes occur:
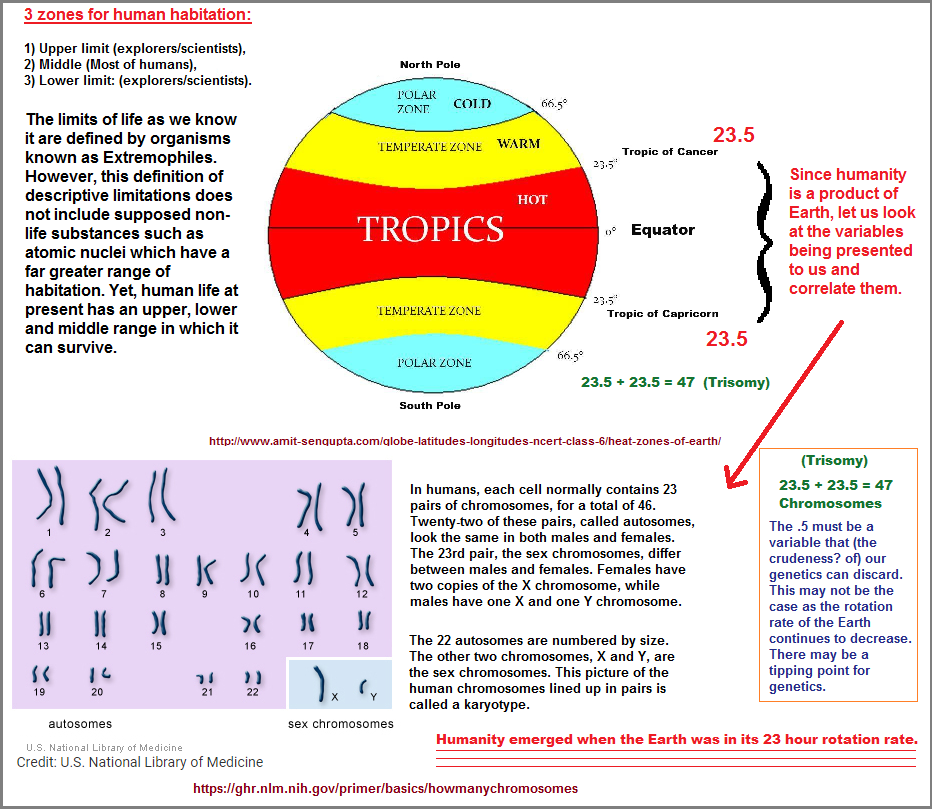
While there are different classes of Extremophiles based on the type of extreme environment in which they thrive, the classes may be viewed as sub-divisions under the three heading of higher, lower... and an unreferenced central position. Notice that the words "high" and "below" are used but no reference to the centrality of most life forms... though an oxygen environment (as opposed to a reduced oxygen environment), is an example of extremism in the sense it is a toxic environment to those life forms existing primarily without an oxygen atmosphere. Here are some examples of Extremophiles:
- Acidophile: an organism that thrives in acidic environments with pH levels of 3 and below.
- Alkaliphile: an organism that thrives in alkaline environments with pH levels of 9 and above.
- Barophile: an organism that lives in high pressure environments, such as deep-sea habitats.
- Halophile: an organism that lives in habitats with extremely high salt concentrations.
- Hyperthermophile: an organism that thrives in environments with extremely high temperatures; between 80–122 °C or 176-252 °F.
- Psychrophile: an organism that survives in extreme cold conditions and low temperatures; between -20 °C to +10 °C or -4 °F to 50 °C.
- Radiophile: an organism that thrives in conditions with high levels of radiation, including ultraviolet and nuclear radiation.
- Xerophile: an organism that lives in extreme dry conditions.
Most extremophiles are microbes that come from the world of bacteria, Archaea, protists, and fungi. Larger organisms such as worms, frogs, insects, crustaceans, and mosses also make there homes in extreme habitats.
by Regina Bailey, May 23rd, 2019
Note: If it is not already clear to some readers, let me reiterate the thought processing I am using: The reason for defining the upper limit of oxygen (which can involve water because of the H2O configuration) is to create a working puzzle with different inter-locking pieces. I am creating the puzzle of an inter-active life domain leading up to the disposition of a "threes" perspective being used in our many different kinds of interpretations, (along with the acknowledgement of it curious absence); to precipitate a presumed parameter of all considerations... though they are presently dynamic and fluidic and contour malleable... not to mention being described with various types of code (language, symbols, non-language, bits and pieces, etc...)
A timeline involving the cataloguing of enumerated molecular values will point out that there exists a repetition of low molecular number values with higher values apparently occurring with non-biologically important applications. In other words, take a look at the chemical formulas of the following biological processes to note that the numerical values all exhibit low numbers. We do not see for instance (as an exaggerated example) something as: C1698710 H4436954 O643228973, etc., for basic biological processes. Just because we use the word "basic" or "fundamental" or "primitive" doesn't mean the enumeration has to be low in values... thereby initiating an inclination that such words are enough of an explanation and we need not question any further.
No less, when constructing a timeline with multiple variables which can affect (even effect) life, let us begin developing a list which describes limitations, though different readers may be awestruck by any one (or more) of them. The limitations being described are expressions of human perception and cognition. This may not be the actual case but it is a beginning reference:
- Meteorological phenomena (rain, wind, lightning, tornadoes, snow, etc...)
- astronomical phenomena (stars, meteorites, asteroids, sun, moon... planetary...)
- geological phenomena (earth quakes, volcanoes, plate techtonics, etc...)
- Atmospheric phenomena
- Chemical processes
- Atomic processes
- Biological processes
- Religion, Philosophy, Government, Economics, Mathematics, Science, Art, Music, Athletic ability, etc...
To take but one example from the above list, in terms of what kind of government is used by humans... and those used by different types of life forms in their life styles (such as bees, ants, termites, birds, etc...), there are different types but not all of them are routinely used. In short, they are pretty simplistic cognitive strategies which can be compiled under a 3-to-1 ratio profile having a variety of names. The following image is illustrative but wholly insufficient in providing a list of government types, though some labels are best used as headings under which multiple ideas can be placed. Let me list a few more following the image:

Forms of Government
Table of Contents
- Anarchy
- Aristocracy
- Bureaucracy
- Capitalism
- Colonialism
- Communism
- Democracy
- Federalism
- Feudalism
- Kleptocracy
- Meritocracy
- Military Dictatorship
- Monarchy
- Oligarchy
- Plutocracy
- Republicanism
- Socialism
- Theocracy
- Totalitarianism
- Tribalism
Here are a few other examples of different government types... if not in function, then in what the name is assumed to imply:
- Cenocracy (Means "New Government". Once you get past some of the personalized orientations, you can find some interesting
- ideas such as a government formula practicing a "government of one's peers" ideology required by the Constitutional mandate for getting a full measure of
- Inalienable Rights.)
- Confederation
- Constitutional (with or without a Constitution, Bill of rights, etc...)
- Corporatocracy
- Democracy (types of different democracy models can be found on this page)
- Dictator
- Emperor
- Equality (not typically labeled a government but a governmental practice like humanitarian)
- Federation
- Junta
- Libertarianism
- Marxian (wayward compilation of socialist/communist ideology)
- Matriarchialism
- Tyranny Governments Types: Kinds & Varieties
And here is another List of government forms, some of which give the impression of being an exercise in intellectual playfulness (and a repeat of those already mentioned):
- Anarchism
- 2 Anarcho-capitalism
- 3 Anarchy
- 4 Androcracy
- 5 Aristocracy
- 6 Autocracy
- 7 Capracracy
- 8 Communism
- 9 Corporatocracy
- 10 Demarchy
- 11 Democracy
- 12 Despotism
- 13 Dictatorship
- 14 Epistemocracy
- 15 Ethnocracy
- 16 Exilarchy
- 17 Fascism
- 18 Feudalism
- 19 Futarchy
- 20 Geniocracy
- 21 Gerontocracy
- 22 Kakistocracy
- 23 Kleptocracy
- 24 Kratocracy
- 25 Kritocracy or Krytocracy
- 26 Matriarchy
- 27 Meritocracy
- 28 Minarchy
- 29 Mobocracy or ochlocracy
- 30 Monarchy
- 31 Necrocracy
- 32 Oligarchy
- 33 Panarchracy
- 34 Patriarchy
- 35 Plutocracy
- 36 Republic
- 37 Socialist republic or people's republic
- 38 Stratocracy
- 39 Technocracy
- 40 Thalassocracy
- 41 Theocracy
- 42 Theodemocracy
- 43 Timocracy
- 44 Tyranny
Origination date: Tuesday, June 25, 2019... 4:41 AM
Initial Posting: Friday, August 30, 2019... 5:57 AM
Updated Posting: Tuesday, January 17, 2023... 11:49 AM
Herb O. Buckland
herbobuckland@hotmail.com