~ 3 ~
~ The Study of Threes ~
http://threesology.org
Researchers as of 8/29/2019
| Devil's Advocate Series: | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| 10 | 11 | 12 | 13 | 14A 14B |
15 | 16 | 17 | 18 |
| 19 | 20 | 21 | 22A 22B |
23 | 24 | 25 | 26 | 27 |
| 28 | 29 | 30 A | 30 B | 31 | 32 | 33a | 33b | 33c |
| 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 A | 41 B |
One of the biggest (but not the only) questions concerning life on Earth is how early did it start? Since life as we know it requires water and water has oxygen, the presence of water on the Earth during the formative years billions of years ago, leads us in the direction of describing what we mean by life... now that the Mystery of Earth's Water Origin is supposedly solved. In other words, our present definition of life states that the earliest form(s) of life did not need ATMOSPHERIC levels of oxygen (levels that we humans think are necessary for engaging in the processes of biology), because the Earth had an ocean full of oxygen, albeit tied to Hydrogen, as displayed by the commonly three-patterned formula known H2O. Early organisms had access to oxygen but "chose" not to indulge in the available resource. It took their later-born rebellious youth to experiment with the toxic (oxygen) substance, much like teenagers of today experimenting with different drugs and behaviors. (Naturally occurring oxygen is composed of three stable isotopes, 16O, 17O, and 18O, with 16O being the most abundant (99.762% natural abundance)).
In viewing the adoption of converting to the usage of oxygen by using a "rebellious youth" analogy, we can further the analogy by introducing materials involving percentages and reasons for substance uses, thus describing that oxygen may not have been the only substance that early life forms on Earth "experimented" with, and that the early life forms were also subjected to reasons analogously defined by a chart of rationales for the adopted behaviors. No doubt many early life forms perished by substance abuse and that they early life forms alternatively came from abusive or loving home environments, though the larger surrounding environment may not have been so. If the analogy I am using is not too much of a metaphor for you, then the idea of "rebellious life forms" perspective is a comparison that will not be too difficult to grasp, so long as representative pairing of "substances" and "experimentation" are adequately adjusted to fit within the respective life-form cultures. (If one has ever ventured into a social setting where people were permitted to smoke, one easily gets to witness what is meant by an environment with a low or absence of oxygen. Tobacco and other substances being smoked is the human-related expression of a reduced oxygen environment, just as is the usage of chemicals polluting the air). Is humanity trying to regress to an early biological stage of development where environmental conditions were quite different from later oxygen rich environments? Rebellious life forms billions of years ago may have lots of things in common with the rebellious human life forms of today.

|
History of Earth and the Early Atmosphere |
Evolution and Oxygen |
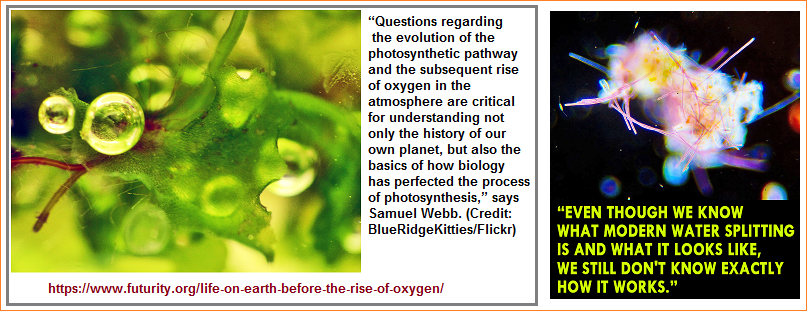 CALTECH (US) One of science's greatest mysteries is how and when the process responsible for producing oxygen on Earth through the splitting of water molecules first began. Now, a team led by geobiologists at the California Institute of Technology (Caltech) has found evidence of a precursor photosystem involving manganese that predates cyanobacteria, the first group of organisms to release oxygen into the environment via photosynthesis. The findings, outlined in the Proceedings of the National Academy of Sciences, strongly support the idea that manganese oxidation which, despite the name, is a chemical reaction that does not have to involve oxygen provided an evolutionary stepping-stone for the development of water-oxidizing photosynthesis in cyanobacteria. "Water-oxidizing or water-splitting photosynthesis was invented by cyanobacteria approximately 2.4 billion years ago and then borrowed by other groups of organisms thereafter," explains Woodward Fischer, assistant professor of geobiology at Caltech and a coauthor of the study. "Algae borrowed this photosynthetic system from cyanobacteria, and plants are just a group of algae that took photosynthesis on land, so we think with this finding we're looking at the inception of the molecular machinery that would give rise to oxygen." Before the rise of oxygen Photosynthesis is the process by which energy from the sun is used by plants and other organisms to split water and carbon dioxide molecules to make carbohydrates and oxygen. Manganese is required for water splitting to work, so when scientists began to wonder what evolutionary steps may have led up to an oxygenated atmosphere on Earth, they started to look for evidence of manganese-oxidizing photosynthesis prior to cyanobacteria. Since oxidation simply involves the transfer of electrons to increase the charge on an atom and this can be accomplished using light or O2 it could have occurred before the rise of oxygen on this planet. "Manganese plays an essential role in modern biological water splitting as a necessary catalyst in the process, so manganese-oxidizing photosynthesis makes sense as a potential transitional photosystem,†says Jena Johnson, a graduate student in Fischer's laboratory at Caltech and lead author of the study. |
While a non-oxygen atmosphere is toxic to humans and other life forms dependent on oxygen, an oxygenated atmosphere is toxic to those life forms who not only do not require it, but are hurt by the presence of oxygen just as are some cellular events in our bodies can be aided or hurt by too much or too little oxidation. The ideas of oxidation and anti-oxidation has become a huge market that exploits the public. However, there are some who are interested in the topic enough to seek out literature focused on the issue. Here are some examples of different perspectives:
- Many people are desirous to find a list of foods with antioxidant levels.
- Others are more interested in following the trend of popularity uses of one product or another such as supplementation with supposed anti-oxidant vitamins and minerals.
- Still others prefer an in depth discussion about Antioxidants
- Many people are said to intentionally consume food containing what they believe to be copious amounts of food or after-market products called anti-oxidants, in an attempt to address the issue of oxidative stress on the body.
- Yet there is evidence that for some physical issues, such as (Antioxidant vitamin and mineral supplements for preventing age-related macular degeneration)there is as yet no scientific basis for supplementation.
- And there are a few who strive to Look beyond the commercialized hype of antioxidants
However, the foregoing references necessarily brings us to include the topic of oxygen in our on-going mental (timeline) equation with more than just lip-service, which involves not only when and how atmospheric oxygen began, but to recognize its limits as we are able to identify it today... as it has occurred over billions of years. The mental model of our timeline is like the creation of a puzzle we are attempting to lay out in front of us by selectively delineating puzzle pieces such as the edges and corners, before tackling the interior picture. The pieces of the puzzle are scattered everywhere in different containers called subjects, and some are "hiding" right out in the open as part of a given culture's traditions and day-to-day conventions of language. Is the overall shape of the puzzle circular? Linear? Triangular? or otherwise? Is it fluidic or dynamic like some picture frames which are capable of changing multiple pictures, or is it static giving the impression of an old photograph, painting, sketch, doodle, graffiti, tattoo, fresco, sculpture, frieze, monument, statue, song, dance or otherwise? Is it one, two, three or more dimensional? Does it have color or is it void of color, sound, reflection-refraction-diffraction/absorption? What are its depths, heights, widths/lengths?
Indeed, within the range of present day possibility, we need to know ranges of occurrence and ranges of absence, holes, intermittancy, etc.. While the following chart provides an indication of oxygen's upper limits in the atmosphere, it does not likewise describe its depths nor widths. Oxygen is one of the multiple variables we need to consider in our timeline equation which will provide an indication of boundaries (limitations):
| 29,000 ft | 8,839 m | 6.9 % | Ultra | Mt. Everest (29,029') |
| 28,000 ft | 8,534 m | 7.2 % | Ultra | K2 (28, 251') |
| 27,000 ft | 8,230 m | 7.5 % | Ultra | |
| 26,000 ft | 7,925 m | 7.8 % | Ultra | |
| 25,000 ft | 7,620 m | 8.1 % | Extreme | |
| 24,000 ft | 7,315 m | 8.4 % | Extreme | |
| 23,000 ft | 7,010 m | 8.7 % | Extreme | Aconcagua (22,841') |
| 22,000 ft | 6,706 m | 9.0 % | Extreme | |
| 21,000 ft | 6,401 m | 9.4 % | Extreme | |
| 20,000 ft | 6,096 m | 9.7 % | Extreme | Denali (20,308') |
| 19,000 ft | 5,791 m | 10.1 % | Extreme | Kilimanjaro (19,341') |
| 18,000 ft | 5,486 m | 10.5 % | Extreme | |
| 17,000 ft | 5,182 m | 11.0 % | Very High | |
| 16,000 ft | 4,877 m | 11.4 % | Very High | Mont Blanc (15,781') |
| 15,000 ft | 4,572 m | 11.8 % | Very High | |
| 14,000 ft | 4,267 m | 12.3 % | Very High | Pikes Peak (14,115') |
| 13,000 ft | 3,962 m | 12.7 % | Very High | |
| 12,000 ft | 3,658 m | 13.2 % | High | Mt. Baldy (12,441') |
| 11,000 ft | 3,353 m | 13.7 % | High | Mt. Phillips (11,711') |
| 10,000 ft | 3,048 m | 14.3 % | High | |
| 9,000 ft | 2,743 m | 14.8 % | High | |
| 8,000 ft | 2,438 m | 15.4 % | High | Aspen, CO (8,000') |
| 7,000 ft | 2,134 m | 16.0 % | Medium | |
| 6,000 ft | 1,829 m | 16.6 % | Medium | Mt. Washington (6288') |
| 5,000 ft | 1,524 m | 17.3 % | Medium | Boulder, CO (5328') |
| 4,000 ft | 1,219 m | 17.9 % | Medium | |
| 3,000 ft | 914 m | 18.6 % | Medium | |
| 2,000 ft | 610 m | 19.4 % | Low | |
| 1,000 ft | 305 m | 20.1 % | Low | |
| 0 ft | 0 m | 20.9 % | Low | Sea Level |
| Altitude (feet) | Altitude (meters) | Effective Oxygen % | Altitude Category | Example |
To feel in the blanks in the above chart with respect to the height of mountains, you may like:
- List of the highest major summits of North America
- The Tallest Mountains In The South American Andes
- List of highest mountain peaks of Africa
- TOP 10 Highest Mountains in Asia
- List of highest points of European countries
- Tallest Mountains in Russia
Origination date: Tuesday, June 25, 2019... 4:41 AM
Initial Posting: Thursday, August 29, 2019... 5:06 AM
Updated Posting: Tuesday, January 17, 2023... 11:47 AM
Herb O. Buckland
herbobuckland@hotmail.com